
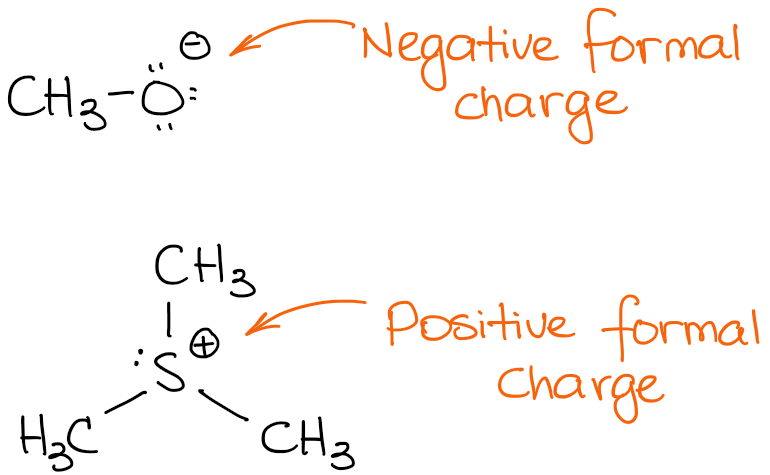
This difference in practice stems from the relatively straightforward assignment of bond order, valence electron count, and hence, formal charge for compounds only containing main-group elements (though oligomeric compounds like organolithium reagents and enolates tend to be depicted in an oversimplified and idealized manner), while there are genuine uncertainties, ambiguities, and outright disagreements when these assignments are attempted for transition-metal complexes.įormal charge compared to oxidation state The third structure, on the other hand, follows the "inorganic" convention, and only the total charge is given. In the second structure, the L-type ligand is depicted with a coordinate or "dative" bond to avoid additional formal charges. The first two follow the "organic" convention, by showing formal charges. Three different depictions of the charges on trichloro(triphenylphosphine)palladium(1-). Instead a top-right corner ⌝ will be drawn following the covalently-bound, charged entity, in turn followed immediately by the overall charge. On the other hand, many workers in organometallic and a majority of workers in coordination chemistry will omit formal charges, unless they are needed for emphasis, or they are needed to make a particular point. They may or may not be enclosed in a circle for clarity. Formal charges are drawn in close proximity to the atom bearing the charge. In contrast, this convention is not followed in inorganic chemistry. In organic chemistry convention, formal charges are an essential feature of a correctly rendered Lewis–Kekulé structure, and a structure omitting nonzero formal charges is considered incorrect, or at least, incomplete. The formal charge system is just a method to keep track of all of the valence electrons that each atom brings with it when the molecule is formed. It is important to keep in mind that formal charges are just that – formal, in the sense that this system is a formalism. The formal charges computed for the remaining atoms in this Lewis structure of carbon dioxide are shown below.Subtract the number of electrons in the circle from the number of valence electrons of the neutral atom in isolation (in its ground state) to determine the formal charge.Count up the number of electrons in the atom's "circle." Since the circle cuts the covalent bond "in half," each covalent bond counts as one electron instead of two.Draw a circle around the atom for which the formal charge is requested (as with carbon dioxide, below).Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge = 0)Įven though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all.Carbon single bonded to one oxygen and double bonded to another (carbon = +1, oxygen double = 0, oxygen single = −1, total formal charge = 0).Carbon single bonded to both oxygen atoms (carbon = +2, oxygens = −1 each, total formal charge = 0).

There are different ways to draw the Lewis structure Example: CO 2 is a neutral molecule with 16 total valence electrons.It can also be found visually as shown below. Where V is the number of valence electrons of the neutral atom in isolation (in its ground state) L is the number of non-bonding valence electrons assigned to this atom in the Lewis structure of the molecule and B is the total number of electrons shared in bonds with other atoms in the molecule.


 0 kommentar(er)
0 kommentar(er)
